fe3+ electron configuration|full electron configuration for fe : Manila Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s . August Palang Pero Nagmano na Agad si Inaanak Kay Ninong . 04:34 HD. Ang Huling Kalikot ni Lolo Bobot
PH0 · iron electron configuration
PH1 · full electron configuration for fe
PH2 · fe2+ electron configuration
PH3 · fe 3+ electron configuration
PH4 · electron configuration of v3+ ion
PH5 · electron configuration for nitrogen
PH6 · electron configuration for fe2+ ion
PH7 · co3+ ground state
PH8 · Iba pa
Sveits er landet med lavest Eurovision odds blant årets deltakere. Landet har deltatt i Melodi Grand Prix hele 63 ganger og står med to seire i konkurransen – i 1956 og 1988. En av de mest kjente artistene i Sveits sin Eurovision-historie er Lys Assia, som var landets bidrag i de tre første årene av konkurransen (1956–1958).
fe3+ electron configuration*******Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period .Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 .Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .Therefore the N electron configuration will be 1s 2 2s 2 2p 3. Video: Nitrogen .fe3+ electron configurationIn order to write the Mg electron configuration we first need to know the .
Electron Configuration Notation:-shows the arrangment of electrons around the .Electron Configuration Notation:-shows the arrangment of electrons around the .
In order to write the Argon electron configuration we first need to know the .Therefore the O electron configuration will be 1s 2 2s 2 2p 4. Video: Oxygen . 5.3K. 705K views 10 years ago. The electron configuration for Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 The electron configuration for Fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5 .more. Fe has the electron configuration [Ar] 3d^6 4s^2, Fe2+ has the electron configuration [Ar] 3d^6, and Fe3+ has the electron configuration [Ar] 3d^5. The .The complete electron configuration of Fe3+is 1s2 2s2 2p6 3s2 3p6 3d5. Fe2+have 13 valence electrons in s, p and d orbitals. The distribution of electrons is as 2 electrons .fe3+ electron configuration full electron configuration for fe FAQ. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine . This chemistry video tutorial explains how to find the electron configuration of Ions such as Mg2+, P3-, Fe2+, and Fe3+.Electron Configuration - Basic Intro:.
There are allotropic forms of iron and are termed as alpha, delta and gamma iron. Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. The electronic .Electron Configuration of Fe2+ and Fe3+ | Channels for Pearson+. with Jules. Get help from an AI Tutor. Ask a question to get started. Next video. General Chemistry 10. .The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons. As stated, the .
2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of . Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, .
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is . This chemistry video tutorial explains how to find the electron configuration of Ions such as Mg2+, P3-, Fe2+, and Fe3+.Electron Configuration - Basic Intro:.
Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The next six electrons will go in the 2p orbital.
What is the electron configuration for the Fe3+ ion? Please explain why a.) [Ne]3s23p10 b.) [Ar]4s13d6 c.)[Ar]4s03d7 d.) [Ar]4s23d9 e.)[Ar]4s03d5; This problem has been solved! You'll get a detailed solution from a subject matter expert .
The correct option is B 1s2,2s22p6,3s23p63d5. 1s2,2s22p6,3s23p63d6,4s2 is the correct electronic configuration of Fe atom. When iron turns into Ferric ion F e3+, it looses three electrons and becomes positive ion therefore the 3+ sign. Electrons are lost from the outermost orbit first i.e from 4s2, 2 electrons are lost and then from 3d6, 1 . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶. Fe3+ Edelgas Elektronenkonfiguration. Wenn 3 Elektronen von einem neutralen Fe-Atom entfernt werden, wird ein Fe3+-Ion gebildet. Das Edelgas fe3+ Elektronenkonfiguration ist 1s2 2s2 2p6 3s2 3p6 3d5. Zuerst werden 1 Elektronen aus dem 2s-Orbital entfernt, da es eine höhere Energie als das 4d-Orbital hat, und dann wird 3 .March 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also .

Electronic Configuration of Iron. The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a transition metal from group 8 of the periodic table’s first transition series. It is the .
The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .
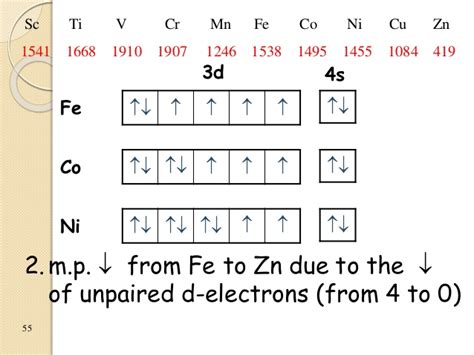
In the ground-state electron configuration of Fe^3+. how many unpaired electrons are present? Express your answer numerically as an integer. Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top or the tool to add orbitals in order of increasing energy, starting at the energy orbitals. El estado fundamental fe3 + la configuración electrónica es 1s2 2s2 2p6 3s2 3p6 3d5. Los primeros 2 electrones se eliminan del orbital 4s porque tiene una energía más alta que el orbital 3d y luego se elimina 1 electrón del orbital 3d, lo que hace que la configuración electrónica de la capa de cenefa sea fe3 + es 3d5.
full electron configuration for fe1s^2\2s^2\2p^6\3s^2\3p^6\3d^5 Iron(III) is the ion of iron in its 3+ oxidation state, meaning that it has lost 3 electrons from its valence shell. The standard electron configuration for an iron atom is: 1s^2\2s^2\2p^6\3s^2\3p^6\3d^6\4s^2 Check to see if the total electrons add up to the proton number of the iron, which is 26. Now, for iron to lose three .
The electron configuration of thallium ion(Tl 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. This electron configuration shows that the thallium ion(Tl 3+) has five shells and the last shell has eighteen electrons and it achieves stable electron configuration. Thallium atom exhibit +1 and +3 oxidation states.
Barangka Ilaya Barangay Hall Barangka Ilaya Barangay Hall is a town hall in Mandaluyong, Eastern Manila District, Metro Manila located on Pinatubo Street. Barangka Ilaya Barangay Hall is situated nearby to Guadalupe Bridge and the scenic viewpoint Makati City Boundary Marker.
fe3+ electron configuration|full electron configuration for fe